NEWS
INDIETROCulminating in 2016 the research activity funded from Fondazione Ginevra Caltagirone on pediatric AML

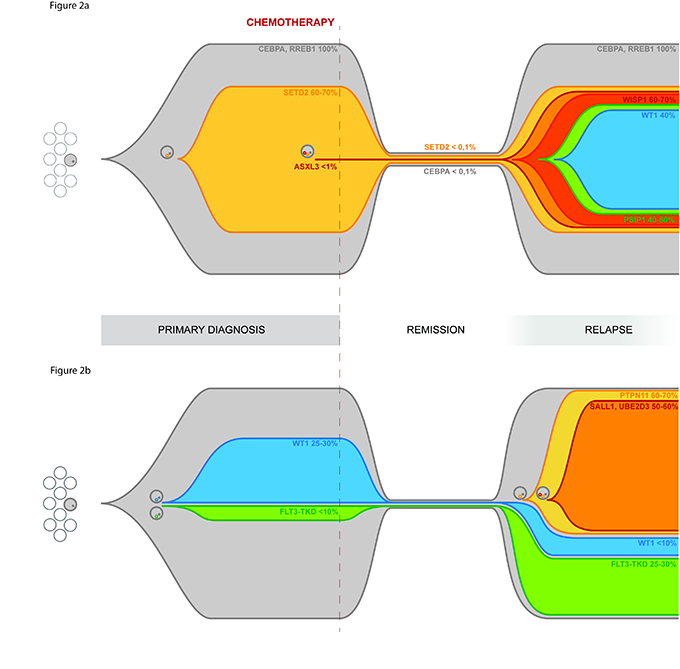
The work on the clonal evolution of AML has continued in recent months of 2016 trying to describe in an almost "graphic" The behavior of this disease when it recurs after chemotherapy. The results of the work to be described below have been recently submitted to the scientific journal Oncotarget and currently under review.
The massive exome sequencing of onset conditions, remission and relapse allowed to precisely define the spectrum of mutations present at onset and compare it with that present at relapse.
A first result was therefore the description of polyclonal pediatric AML, extending the validity of the clonal evolution model of the disease, already validated for adult AML.
A second result was the characterization of mutations present at the onset or recurrence, or both stages of the disease.
Among these is the A72V mutation of PTPN11, which appears to relapse in 64% of the blast population, and the D835E mutation of FLT3, associated with increased cellular proliferative signals, but also mutations described for the first time in pediatric AML including the mutation of TYK2 and ASXL3.
Having thus defined the mutational spectrum of the patients studied in the second part of the project we focused on understanding which of these mutations is important for the development of recurrence and whether mutations present at relapse may be present in a small percentage of clone already all debut.
Ultimately it can be said that many of these mutations are potential targets for targeted therapies, so the accurate molecular characterization of the disease, even at relapse, offers the possibility of adopting targeted therapeutic choices, possibly directed simultaneously toward different subclones. Also from this work it shows the importance of the high-resolution sequencing as potential tool in diagnosis to detect specific mutations for which there are target therapies.
During the second quarter the operations focused to translate the results seen in cell lines derived directly blasts from pediatric patients with acute myeloid leukemia (AML) and positive for the fusion gene CBFA2T3-GLIS2. At the moment the final results unique to a patient tested positive for the fusion gene. The blast cells of this patient together in the blast cells of AML patients negative for the stretched fusion gene were treated with escalating doses of GANT61 for 72 hours and was so obtained the IC50 (lethal dose for 50% of the cells).
Analysis of the data is clearly seen that positive blast cells for fusion of GLIS2 are more sensitive to treatment, compared to negative blasts for the same fusion. This confirms what has been seen in cell lines and suggests likely the drug's specificity in inhibiting the fusion gene.
These data are of great interest because it could open up new therapeutic possibilities combined with other targeted drugs or in combination with conventional chemotherapy. We are now awaiting the comments of the reviewers of Oncotarget magazine and we hope that this research, which involved for these years Dr. Riccardo Masetti and his staff, could pave the way for the inclusion of new drug for the treatment of acute myeloid leukemia in children.

