NEWS
INDIETROPublished the 2015 Report on the scientific research activity funded by Fondazione Ginevra Caltagirone

The line of research developed in 2015 by Dr. Masetti is part of a broader planning already started in 2012, thanks to the support of Caltagirone Foundation, focused on deep sequencing of the genome of pediatric patients with acute myeloid leukemia, still a very aggressive disease and now burdened with a disease-free survival at 5 years still does not exceed 65%.
The objective, as explained above, is to threefold of:
1) identify new molecular markers for diagnosis and disease monitoring,
2) understanding the biological behavior of the disease and its evolution,
3) identify molecular targets that may be susceptible to an integrated targeted biological treatment to conventional chemotherapy.
The
first years of the project focused mainly on the first point.
mutations have been identified in genes that regulate cell
proliferation and cell death, in genes that code for enzymes that
serve to DNA to divide, and others that serve for transcribing the
DNA, in subgroups of AML in particularly unfavorable prognosis.
The
ultimate fusion gene identified in 2015 shall be those resulting from
NUP98 and PHF23 genes and was published in the Journal of Hematology
and Oncology. For the first time, in the article mentioned, it is
demonstrated recurrence in approximately 3% of all AML in normal
karyotype of this fusion gene.
In
2015, in addition to having produced this important result in the
identification of new markers, they were also achieved significant
results with respect to the understanding of the biological behavior
of the disease and the testing of new pharmacological agents targeted
at specific molecular targets of the disease.
The
massive exome sequencing of onset conditions, remission and relapse
of four pediatric patients with AML with normal karyotype has
identified mutations in each present only debut, only to relapse and
mutations present in both of the same patient samples . These results
confirm an eminently polyclonal nature of pediatric AML, extending
the validity of the clonal evolution model of the disease, already
validated by Ding et al for adult AML. It thus configures the
evidence of a genomic complexity considerably higher than previously
thought. In particular, while one or a few mutational events are
initially responsible for the leukemic transformation (primary
events), following the neoplastic population differs in subclones
through the acquisition of additional mutations (secondary events).
The evolution of the disease is then the effect of dynamic
interaction of these mutations with the microenvironment and
treatment to which the patient is subjected.
Among
secondary events highlighted in one of the analyzed patients, include
the A72V mutation of PTPN11, which appears to relapse in 64% of the
blast population, and the D835E mutation of FLT3 clone detected in a
small (6%) at the onset that goes expanding the relapsed (26%). These
events, known to be associated with an increase in cellular
proliferative signals, although not appear decisive in the onset of
the disease, certainly favor its perpetuation and survival to
traditional therapies.
In
the light of the availability of specific pharmacological inhibitors,
stressing the need to characterize the molecular point of view not
only the disease onset, as practiced routinely, but also to
recurrence, to use an individualized therapy, just after bankruptcy
conventional therapies.
Although
the mutations of WT1, a transcription factor particularly expressed
in leukemia cells and mutated in approximately 10% of AML, they are
highly unstable revealed during the course of disease, both in terms
of acquisition of that loss, reflecting the role not primary and
still obscure these events in the pathogenesis of AML. On
the contrary, the CEBPA mutation, a transcription factor mutated in
4.5% of pediatric AML, is present in homozygosity in the totality of
the leukemic population is at the onset that the recurrence of one of
the patients analyzed, in accordance with the primary role attributed
to this event so that the LAM with biallelic mutation CEBPA is
inserted as a provisional entity in the WHO classification of AML.
Other
molecular events that we have identified as possibly contributing to
recurrence of the disease, including a mutation of the tyrosine
kinase TYK2, associated with an increased resistance to apoptosis,
and a SETD2 mutation, a methyltransferase involved in mismatch repair
whose inactivation is associated with a accumulation of somatic
mutations, mechanism able to further increase the genomic complexity
of the disease and promote its plasticity, and then the survival to
therapies.
The
drug used in the study is funded GANT61 an inhibitor of the GLI
family proteins used in preclinical in some solid tumors such as
neuroblastoma that the Hedgehog pathway is abnormally active.
During
the first four months l 'research has focused primarily in
preclinical research models suitable for testing the efficacy of the
drug. In this case, two cell lines were chosen: the M07 and WSU-AML
granted by St. Jude Children's Research Hospital, Memphis in addition
to a series of negative AML cell lines for fusion gene CBFA2T3-GLIS2.
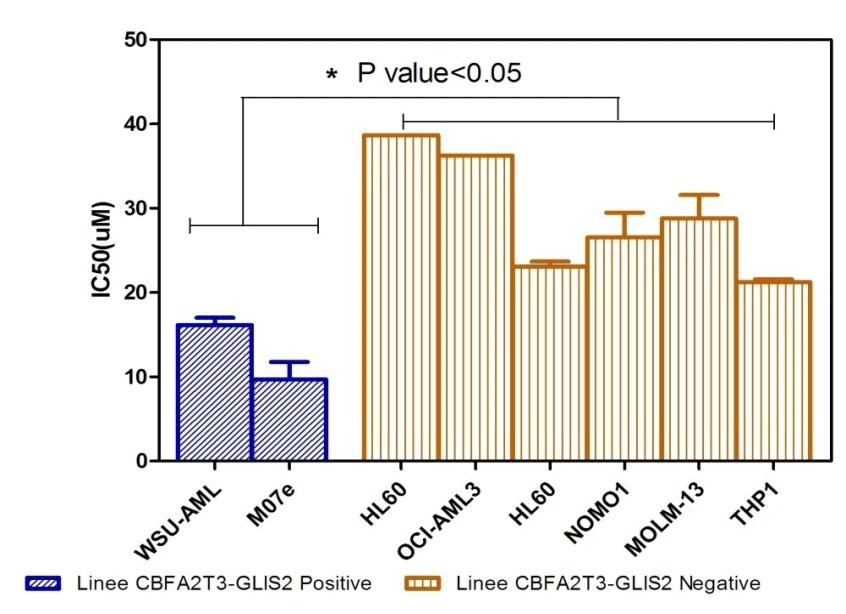
The
positive and negative cell lines to the translocation in question
have been treated for 72h with the 'inhibitor of the pathway
Hedgeogh, the GANT61. 8 were tested concentrations of the drug from
which it is derived the average dose that kills 50% of the cells
(IC50). From the statistical analysis it showed that the positive
cell lines translocation of GLIS2 are more sensitive to treatment
with GANT61 than other negative LAM lines (Figure 1A).
This
was an important result because it has already given a first idea on
the mechanism of action of the drug that acts in some way on the
specifically chosen target (in this case GLIS2 that is indispensable
for the process of leukemogenesis) and at the same time the
non-specific toxicity is low for the other cell lines in which it is
not present the same translocation of GLIS2 same.
In
the same way by treating with the drug the same cell lines with GLIS2
translocation of the gene was conducted a study of gene expression in
order to determine the pharmacological effect on the transcriptional
activity mediated by GLIS2. The two lines were treated with GANT61
for 48h, then was extracted the total RNA of the cell and the gene
expression was quantified through affymetrix platform. Analysis of
the data was confirmed the activation of some specific genes of
Hedgeogh signaling pathway as BMP2, GATA3, CCND2 or NCAM1 cited in
the two previous studies, some of which undergoes a clear decrease of
the expression following treatment pharmacological. But in addition
to these genes the statistical analysis of the data showed for the
first time the expression of other genes with important functions
within the neoplastic cell. Among
these were some involved in the regulation of proliferation processes
(kIF14, MELK, MCM10, NUF2), cell cycle regulation (CCNA2, CDKN3,
CDC7, PRC1), repair (BRCA1, BRCA2) and especially genes involved in
epigenetic processes such as methylation DNA (DNMT1, DNMT3B). All of
these genes undergo a statistically significant drop following
treatment with GANT61.
 This
is a very interesting result as it helps to better understand tumor
biology of this subgroup of AML. The decrease of the expression of
all these genes after treatment with GANT61 strengthens the
hypothesis that the drug is specifically for the aberration in
question and above could be a first-line treatment in the treatment
of this subgroup of AML with poor prognosis. In addition, since the
drug acts negatively on the expression of genes involved in DNA
repair mechanisms, the GANT61 may potentiate the effect of
conventional chemotherapeutic agents used today in the treatment
protocols.
This
is a very interesting result as it helps to better understand tumor
biology of this subgroup of AML. The decrease of the expression of
all these genes after treatment with GANT61 strengthens the
hypothesis that the drug is specifically for the aberration in
question and above could be a first-line treatment in the treatment
of this subgroup of AML with poor prognosis. In addition, since the
drug acts negatively on the expression of genes involved in DNA
repair mechanisms, the GANT61 may potentiate the effect of
conventional chemotherapeutic agents used today in the treatment
protocols.

